Kerry announces licensing agreement for rice-derived postbiotic
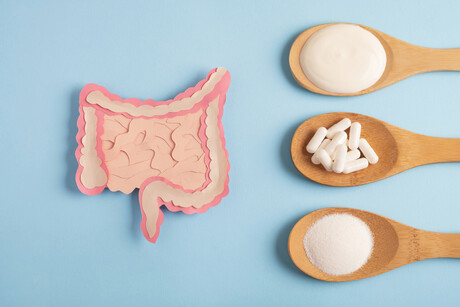
Kerry has announced a commercial agreement with Japan-based Kameda Seika to market and distribute the company’s rice-derived postbiotic across a range of applications in Europe and the Americas.
Kameda Seika is a global rice cracker company that has researched rice-based lactobacillus for over 25 years. Its postbiotic Lactobacillus K-1 (Lactobacillus casei subsp, 327) is claimed to improve both digestive and skin health and can be used in food, beverages and supplements.
Postbiotics are the metabolic by-products of fermentation. They offer similar functionality to probiotics and are backed by growing clinical evidence. Manufacturers may use them as a heat- and acid-stable health-enhancing ingredient.
Kerry will leverage Kameda’s technology portfolio, application expertise and commercial footprint to drive the commercialisation of the technology to reach users outside of Japan.
Lactobacillus K-1 was tested in a randomised, double-blind, placebo-controlled study which found that it may improve digestive health. Results were further supported by additional scientific data which found that it may improve gut and skin health.
Bird flu at Mainland's Otago farm, no disease yet on other farms
About 1000 samples were received for testing at the Ministry for Primary Industries' (MPI)...
Burcon, Puratos partner for innovative canola protein applications
Burcon NutraScience Corporation, a global technology player in the development of plant-based...
Oily fish, fruits and beer can cut rheumatoid arthritis risk
Moderate alcohol consumption and a higher intake of fruits, oily fish, and cereals are linked to...










